Abstract
Sickle cell anemia (SCA) is an inherited red blood cell disorder that leads to hemoglobin polymerization when it deoxygenates. Lung function abnormalities are observed in up to 90% of adults with SCA (PMID: 16556694) and both the prevalence (PMID: 20644948) and rate of FEV1 decline (PMID: 18383325) are greater in SCA adults compared to the general population. Furthermore, low FEV1 is an independent predictor of early mortality in SCA (PMID: 26261241). Understanding the risk factors and mechanisms for abnormal lung function, which can impact blood oxygen and pH levels, is especially important in SCA.
We evaluated the clinical, laboratory, and genetic risk factors for abnormal pulmonary function in a retrospective cohort of SCA patients. Pulmonary function test (PFT) abnormalities were categorized based on the American Thoracic Society/European Respiratory Society Guidelines. Alpha-thalassemia and BCL11A rs1427407 status were determined by PCR. Continuous and categorical variables were compared using the Kruskal-Wallis and Chi-square test, respectively, and Kaplan-Meier curves and Cox proportional hazards models were used to compare survival patterns. Median and interquartile ranges (IQR) are provided.
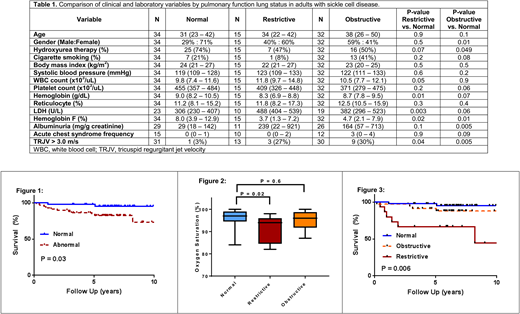
Between 12/2004 and 12/2015, 90 SCA adult patients had PFTs performed as part of their routine medical care. The median age of the cohort was 37 years (IQR, 24-47years), 46% were male, 59% were on hydroxyurea, and 28% were cigarette smokers. Abnormal pulmonary function was observed in 56 (62%); 32 (35.6%) had obstructive, 15 (16.7%) had restrictive, and 9 (10%) had mixed lung defects. SCA patients with abnormal PFTs were more anemic (8.4 vs. 9.0g/dL, P=0.004), had higher LDH (441 vs. 306U/L, P=0.002) and urine albumin concentrations (180 vs. 29mg/g creatinine, P=0.004), and a lower hemoglobin F% (4.2% vs. 8.0%, P=0.004) compared to SCA with normal PFTs. SCA patients who inherited the BCL11A rs1427407 T allele had a lower prevalence of abnormal PFTs (G/G: 67%, G/T: 55%, T/T: 50%; P=0.2), but this was not statistically significant, and we did not observe any patterns between co-inheritance of α-thalassemia and abnormal PFTs (αα/αα: 60%, α-/αα: 64%, α-/α-: 60%; P=0.9). On logistic regression analysis, LDH (natural log OR 8.2, P=0.03) was independently associated with abnormal pulmonary function after adjusting for age, gender, and hydroxyurea use. SCA patients with abnormal pulmonary function had earlier mortality compared to those with normal pulmonary function (Figure 1) (log-rank P=0.03; age-adjusted HR 6.1, P=0.09).
Next, we analyzed clinical and laboratory risk factors associated with obstructive or restrictive lung disease. Lower hemoglobin F% and a greater prevalence of tricuspid regurgitant velocity (TRJV) > 3.0m/s were associated with both restrictive and obstructive pulmonary function compared to normal pulmonary function (Table 1). In addition, lower hemoglobin concentration and higher LDH were associated with restrictive lung disease while less common treatment with hydroxyurea and higher urine albumin concentration were associated with obstructive lung disease compared to SCA patients with normal PFTs (Table 1). SCA patients with restrictive lung disease had a lower oxygen saturation, determined by pulse oximetry, compared to those with normal PFTs (P=0.02) while no difference in oxygen saturation was observed between those with obstructive versus normal PFTs (P=0.6) (Figure 2). Mortality risk was significantly increased for SCA patients with restrictive (age-adjusted HR 2.0, P=0.01) but not obstructive lung disease (age-adjusted HR 1.6, P=0.4) compared to those with normal PFTs (log rank P=0.006) (Figure 3).
In conclusion, we demonstrate that increased hemolysis is associated with abnormal pulmonary function based on a lower hemoglobin concentration and lower hemoglobin F% and higher LDH. We also demonstrate that restrictive-type lung disease is associated with a lower oxygen saturation and increased mortality risk compared to those with normal PFTs. Future studies investigating the role of hemolysis in lung disease and therapies to improve pulmonary function, particularly in those with restrictive lung disease, are warranted in SCA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal